Porosity
in
pharmaceutical
materials
can
be
present
in
various
locations:
within
tablet
cores
and
granules,
coatings,
and
within
the
particles
of
a
pre-cursor
powder
blend
and
also
the
void
spaces
between
powder
particles.
The
latter
dictates
the
porous
characteristics
of
tableted
forms.
SSA
measurement
is
routinely
and
usefully
applied
to
the
characterisation
of
powder
samples
but
the
pore
sizes
present
commonly
fall
within
the
range
measured
by
the
mercury
porosimetry
technique.
This
provides
a
complete
picture
of
pore
size,
pore
volume,
volume
porosity
and
density.
It
can
also
provide
insight
to
pore
geometry.
These
porosity
characteristics
then
dictate
performance
behaviours,
may
be
indicative
of
atypical
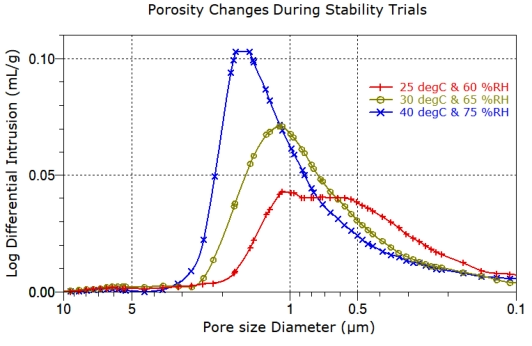
performance, and often change during the ageing processes applied during stability trials.
Tel: 01763 262333
Pharmaceutical Characterisation at MCA Services
The
physical
characteristics
of
pharmaceuticals
profoundly
influence
the
properties
and
effectiveness
of
the
final
dosage
form,
be
it
administered
via
tablet,
granule
/
capsule
or
inhalation
routes.
Furthermore,
knowledge
of
physical
characteristics
often
contributes
to
the
critical
understanding
of
all
components,
such
as
API,
excipients,
powder
blends
and
ribbons
as
well
as
the
final
form.
They
are
also
applicable
to
all
stages
of
pharmaceutical processes: from formulation through manufacturing to quality control, stability trials and trouble-shooting atypical behaviour.
Physical
characteristics
commonly
include
specific
surface
area
(SSA),
pore
size
and
volume
distributions
and
density,
which
together
provide
a
complete
understanding
of
overall
porosity.
In
turn,
porosity
has
direct
effects
on
many
aspects
of
performance,
for
example
dissolution
and
disintegration
rates,
strength
and
hardness
and
long
term
stability
of
solid
dosage
forms.
They
also
influence
formulation
and
effect
stability
of
solid components in many emulsion, paste and topical forms.
Key Applications
Characterisation of API and excipient materials
Characterisation of powder blends
Investigation of dissolution rate
Study of disintegration
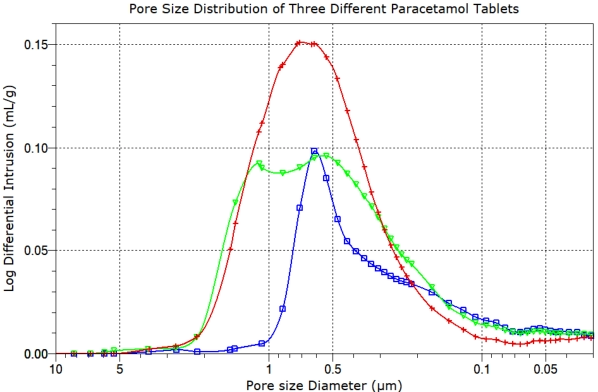
Investigation of batch failures and inter-batch variability
Influence of stability trials on porosity
Determination of long-term stability and shelf life
Investigation and establishment of tableting conditions
Identification of counterfeit batches
Significant to approval processes
All analyses undertaken to ISO 17025 standards
Key Analytical Techniques
Mercury Porosimetry
Pore volume Distribution
Pore Size Distribution
Pore Area Distribution
Absolute Porosity
Bulk and Skeletal Density
Pore Geometry
Permeability Measurement
Applicable to all solid forms
Gas Adsorption
BET Specific Surface Area by Nitrogen
BET Specific Surface Area by Krypton (low SSA)
Micro-pore / Meso-pore characterisation
Pore volume Distribution
Pore Size Distribution
Pore Area Distribution
At
MCA
Services
we
offer
a
suite
of
analytical
techniques
for
the
complete
characterisation
of
pharmaceutical
samples.
This
is
combined
with
the
experience
and
expertise
to
actively
assist
with
the
interpretation
of
results
directly
relating
these
to
performance
characteristics
of
the
material.
We
have
many
years
of
experience
partnering
with
the
pharmaceutical
sector,
considering
a
wide
variety
of
dosage
forms
and
applications,
from
formulation to stability trials and identification of the influence of porosity to materials and batches having atypical behaviour.
Pharmaceutical Characterisation
The
physical
characteristics
of
pharmaceuticals
profoundly
influence
the
properties
and
effectiveness
of
the
final
dosage
form,
be
it
administered
via
tablet,
granule
/
capsule
or
inhalation
routes.
Furthermore,
knowledge
of
physical
characteristics
often
contributes
to
the
critical
understanding
of
all
components,
such
as
API,
excipients,
powder
blends
and
ribbons
as
well
as
the
final
form.
They
are
also
applicable
to
all
stages
of
pharmaceutical
processes:
from
formulation
through
manufacturing
to
quality
control, stability trials and trouble-shooting atypical behaviour.
Physical
characteristics
commonly
include
specific
surface
area
(SSA),
pore
size
and
volume
distributions
and
density,
which
together
provide
a
complete
understanding
of
overall
porosity.
In
turn,
porosity
has
direct
effects
on
many
aspects
of
performance,
for
example
dissolution
and
disintegration
rates,
strength
and
hardness
and
long
term
stability
of
solid
dosage
forms.
They
also
influence
formulation
and
effect
stability
of
solid
components
in
many emulsion, paste and topical forms.
Key Applications
Characterisation of API and excipient materials
Characterisation of powder blends
Dissolution rate study
Disintegration study
Investigation of batch failures and inter-batch variability
Influence of stability trials on porosity
Determination of long-term stability and shelf life
Investigation and establishment of tabletting conditions
Identification of counterfeit batches
Significant to approval processes
All analyses undertaken to ISO 17025 standards
Porosity
in
pharmaceutical
materials
can
be
present
in
various
locations:
within
tablet
cores
and
granules,
coatings,
and
within
the
particles
of
a
pre-cursor
powder
blend
and
also
the
void
spaces
between
powder
particles.
The
latter
dictates
the
porous
characteristics
of
tableted
forms.
SSA
measurement
is
routinely
and
usefully
applied
to
the
characterisation
of
powder
samples
but
the
pore
sizes
present
commonly
fall
within
the
range
measured
by
the
mercury
porosimetry
technique.
This
provides
a
complete
picture
of
pore
size,
pore
volume,
volume
porosity
and
density.
It
can
also
provide
insight
to
pore
geometry.
These
porosity
characteristics
then
dictate
performance
behaviours,
may
be
indicative
of
atypical
performance,
and
often
change
during the aging processes applied during stability trials.
Key Analytical Techniques
Mercury Porosimetry
Pore volume Distribution
Pore Size Distribution
Pore Area Distribution
Absolute Porosity
Bulk and Skeletal Density
Pore Geometry
Permeability Measurement
Applicable to all solid forms
Gas Adsorption
BET Specific Surface Area by Nitrogen
BET Specific Surface Area by Krypton (low SSA)
Micro-pore / Meso-pore characterisation
Pore volume Distribution
Pore Size Distribution
Pore Area Distribution
At
MCA
Services
we
offer
a
suite
of
analytical
techniques
for
the
complete
characterisation
of
pharmaceutical
samples.
This
is
combined
with
the
experience
and
expertise
to
actively
assist
with
the
interpretation
of
results
directly
relating
these
to
performance
characteristics
of
the
material.
We
have
many
years
of
experience
partnering
with
the
pharmaceutical
sector,
considering
a
wide
variety
of
dosage
forms
and
applications,
from
formulation
to
stability
trials
and
identification
of
the
influence
of
porosity to materials and batches having atypical behaviour.
















