Tel: 01763 262333
Isosteric Heat of Adsorption Measurement
The
heat
of
adsorption
is
a
measure
of
the
strength
of
interaction
between
an
adsorbate
gas
or
liquid
and
a
solid
adsorbent
surface.
Adsorption
is
an
exothermic
process
and
the
heat
of
adsorption
is,
therefore,
the
energy
released
during
the
process
of
adsorption.
The
heat
of
adsorption
is
a
fundamental
thermodynamic
feature
of
the
adsorption
process
and
its
value
can
be
utilised
practically
when
developing,
selecting
and
assessing
materials
for
a
specific
adsorption
process
or
application.
For
applications
such
as
adsorbents,
separation
processes,
filtration,
gas
storage
and
catalysis
it
is
often
desirable
to
select
or
develop
materials
with
an
affinity
to
adsorb
a
certain
species.
Knowledge
of
the
heat
of
adsorption
of
the
specific
gas
onto
the
sample
surface
is
then
an
important
consideration
and
is
complimentary
to
knowledge
of
the
porosity
of
the material.
Measurement
of
the
isosteric
heat
of
adsorption
is
a
common
technique
for
the
calculation
of
the
heat
of
adsorption.
At
least
two
adsorption
isotherms
are
measured
at
different
temperatures
from
which
adsorption
isosteres
are
constructed.
The
heat
of
adsorption
is
then
calculated
by
application
of
the
Clausius-Clapeyron
equation
applied
across
a
range
of
adsorption
pressures
common
to
the
recorded
isotherms.
Since
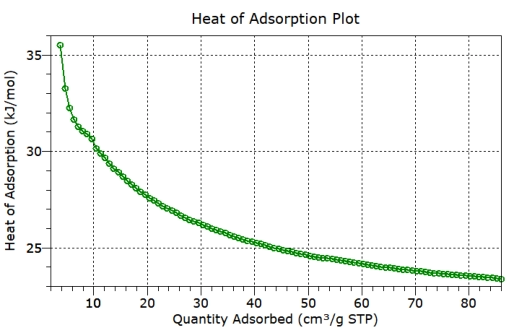
isotherms covering a range of adsorption pressures are recorded, a plot of heat of adsorption as a function of surface coverage is also plotted.
Key Applications and Highlights
Isosteric heat of adsorption measurement
Heat of Adsorption vs Surface Coverage
Heat of adsorption calculated for all data points – low to high surface coverage
Range of adsorbate options available
Measurements from low pressure / surface coverage to saturation
Application of multiple isotherms at different temperatures
Wide range of analytical temperatures
Numerical and graphical results formats
At
MCA
Services
we
use
our
Micromeritics
3Flex
for
heat
of
adsorption
measurements.
A
wide
range
of
analysis
temperatures
can
be
applied
to
the
measurement
of
isotherms.
For
many
applications,
temperatures
close
to
ambient
are
required
and
our
iso-thermal
controller
is
used
to
accurately
maintain
a
stable
temperature
selected
in
the
range
-5
°C
to
+50
°C.
Alternatively,
cryogenic
temperatures
as
low
as
77K
can
be
applied.
For
all
isotherms,
adsorption
commences
at
very
low
relative
pressure
and
is
continued
to
saturation
pressure.
The
heat
of
adsorption
plot
then
represents
low
to
high
surface
coverage:
low
surface
coverage
being
useful
for
further
thermodynamic
calculations
and
higher
surface
coverage representing many applications.
Isosteric Heat of Adsorption Measurement
The
heat
of
adsorption
is
a
measure
of
the
strength
of
interaction
between
an
adsorbate
gas
or
liquid
and
a
solid
adsorbent
surface.
Adsorption
is
an
exothermic
process
and
the
heat
of
adsorption
is,
therefore,
the
energy
released
during
the
process
of
adsorption.
The
heat
of
adsorption
is
a
fundamental
thermodynamic
feature
of
the
adsorption
process
and
its
value
can
be
utilised
practically
when
developing,
selecting
and
assessing
materials
for
a
specific
adsorption
process
or
application.
For
applications
such
as
adsorbents,
separation
processes,
filtration,
gas
storage
and
catalysis
it
is
often
desirable
to
select
or
develop
materials
with
an
affinity
to
adsorb
a
certain
species.
Knowledge
of
the
heat
of
adsorption
of
the
specific
gas
onto
the
sample
surface
is
then
an
important
consideration
and
is
complimentary
to
knowledge
of
the
porosity of the material.
Measurement
of
the
isosteric
heat
of
adsorption
is
a
common
technique
for
the
calculation
of
the
heat
of
adsorption.
At
least
two
adsorption
isotherms
are
measured
at
different
temperatures
from
which
adsorption
isosteres
are
constructed.
The
heat
of
adsorption
is
then
calculated
by
application
of
the
Clausius-Clapeyron
equation
applied
across
a
range
of
adsorption
pressures
common
to
the
recorded
isotherms.
Since
isotherms
covering
a
range
of
adsorption
pressures
are
recorded,
a
plot
of
heat
of
adsorption
as
a
function
of
surface
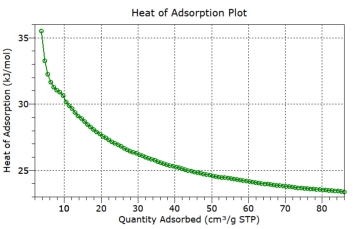
coverage is also plotted.
Key Applications and Highlights
Isosteric heat of adsorption measurement
Heat of Adsorption vs Surface Coverage
Heat of adsorption calculated for all data points – low to high
surface coverage
Range of adsorbate options available
Measurements from low pressure / surface coverage to
saturation
Application of multiple isotherms at different temperatures
Wide range of analytical temperatures
Numerical and graphical results formats
At
MCA
Services
we
use
our
Micromeritics
3Flex
for
heat
of
adsorption
measurements.
A
wide
range
of
analysis
temperatures
can
be
applied
to
the
measurement
of
isotherms.
For
many
applications,
temperatures
close
to
ambient
are
required
and
our
iso-thermal
controller
is
used
to
accurately
maintain
a
stable
temperature
selected
in
the
range
-5
°C
to
+50
°C.
Alternatively,
cryogenic
temperatures
as
low
as
77K
can
be
applied.
For
all
isotherms,
adsorption
commences
at
very
low
relative
pressure
and
is
continued
to
saturation
pressure.
The
heat
of
adsorption
plot
then
represents
low
to
high
surface
coverage:
low
surface
coverage
being
useful
for
further
thermodynamic
calculations
and
higher surface coverage representing many applications.
















