Expertise in the Physical
Characterisation of Materials

MCA Services
Unit 1A Long Barn, North End, Meldreth, Cambridgeshire SG8 6NT UK
01763 262333
© MCA Services




PHARMACEUTICAL ANALYSIS AT MCA SERVICES
The
physical
characteristics
of
pharmaceutical
forms
are
often
critical
to
their
ultimate
effectiveness.
Knowledge,
understanding
and
control
of
physical
properties
is
fundamentally
important
to
every
phase
of
pharmaceutical
formulation,
evaluation,
production
and
QC.
Quantification
of
physical
properties are often classified as being critical within a QbD framework.
The
porous
characteristics
of
pharmaceuticals
in
many
forms,
such
as
APIs,
excipients,
powder
blends,
granules
and
solid
dosages
are
influential
to
pharmaceutical
performance
and
efficiency
and
have
direct
effects
on
dissolution
rate,
solubility,
tablet
strength
and
hardness,
long
term
stability
and
shelf-life.
Key Benefits:
•
Assessment of API and excipient materials
•
Analysis of many pharmaceutical forms
•
Investigation of batch failures
•
Investigation of batch-to-batch variability
•
Determination of long-term stability & shelf-life
•
Identification of counterfeit pharmaceuticals
•
Data interpretation included as standard
•
All analyses undertaken to ISO 17025:2017
•
Adaptable to different test methods
Measurement
of
specific
surface
area
(SSA),
pore
volume,
average
pore
size
and
pore
size
distribution
provides
essential
information
for
the
characterisation
of
the
porous
nature
of
pharmaceuticals.
Porosity
within
pharmaceutical
materials
can
be
present
in
various
locations:
within
tablet
cores
and
coatings,
granules,
pre-cursor
power
blend
particles
as
well
as
the
inter-particle
voids
within
powder
blends.
All
have
profound
effects
on
pharmaceutical
efficiency
and
the
characterisation
of
powder
blends
allows
for
predictions
of
the
solid
dosage
form
to
be
made
and
for
variability
to
be
traced.
Gas
Adsorption
is
typically
used
for
SSA
measurement,
using
either
nitrogen
or
krypton
adsorption.
Mercury
Porosimetry
is
used
for
the
full
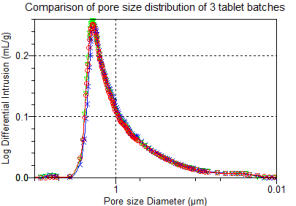
characterisation of pore volume and pore size and is more informative than SSA by gas adsorption data alone.
Key Techniques:
•
BET surface area (SSA) by nitrogen
•
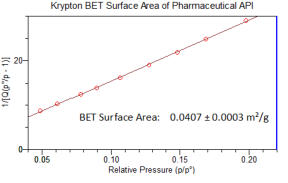
BET surface area (SSA) by krypton for low area samples
•
Mercury Porosimetry for pore volume measurement
•
Mercury Porosimetry for pore size distribution
•
Mercury Porosimetry for volume (%) porosity measurement
•
Micropore analysis for pore volume, area and size measurement
•
Bulk (envelope) density measurement
•
Analysis of powder blends
•
Analysis of solid dosage forms
At
MCA
Services
we
offer
a
complete
suite
of
techniques
for
the
characterisation
of
sample
porosity
with
the
experience
and
expertise
to
actively
assist
with
the
interpretation
of
results
and
their
relation
to
development
and
performance
criteria.
Our
ongoing
in-house
research
continues
to
highlight
the
significance of and application of these analytical techniques to the pharmaceutical sector.
LATEST RESEARCH NEWS:
New research paper by MCA Services in International Journal of Pharmaceutics
BET surface area measurement of commercial magnesium stearate by krypton adsorption in preference to
nitrogen adsorption. International Journal of Pharmaceutics. Volume 568, 10 September 2019.






Expertise in the Physical
Characterisation of Materials
MCA Services
Unit 1A Long Barn, North End,
Meldreth, Cambridgeshire SG8 6NT UK
01763 262333
© MCA Services




PHARMACEUTICAL ANALYSIS
The physical characteristics of pharmaceutical forms are often critical to
their ultimate effectiveness. Knowledge, understanding and control of
physical properties is fundamentally important to every phase of
pharmaceutical formulation, evaluation, production and QC. Quantification
of physical properties are often classified as being critical within a QbD
framework.
The porous characteristics of pharmaceuticals in many forms, such as APIs,
excipients, powder blends, granules and solid dosages are influential to
pharmaceutical performance and efficiency and have direct effects on
dissolution rate, solubility, tablet strength and hardness, long term stability
and shelf-life.
Key Benefits:
•
Assessment of API and excipient materials
•
Analysis of many pharmaceutical forms
•
Investigation of batch failures
•
Investigation of batch-to-batch variability
•
Determination of long-term stability & shelf-life
•
Identification of counterfeit pharmaceuticals
•
Data interpretation included as standard
•
All analyses undertaken to ISO 17025:2017
•
Adaptable to different test methods
Measurement of specific surface area (SSA), pore volume, average pore size
and pore size distribution provides essential information for the
characterisation of the porous nature of pharmaceuticals. Porosity within
pharmaceutical materials can be present in various locations: within tablet
cores and coatings, granules, pre-cursor power blend particles as well as
the inter-particle voids within powder blends. All have profound effects on
pharmaceutical efficiency and the characterisation of powder blends allows
for predictions of the solid dosage form to be made and for variability to be
traced.
Gas Adsorption is typically used for SSA measurement, using either
nitrogen or krypton adsorption. Mercury Porosimetry is used for the full
characterisation of pore volume and pore size and is more informative
than SSA by gas adsorption data alone.
Key Techniques:
•
BET surface area (SSA) by nitrogen
•
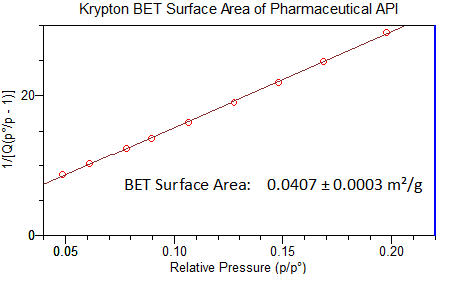
BET surface area (SSA) by krypton for low area samples
•
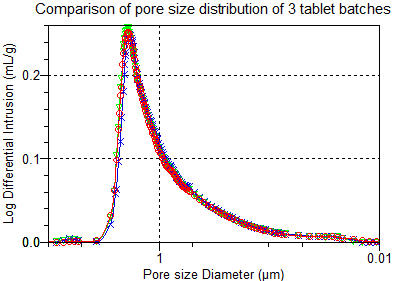
Mercury Porosimetry for pore volume measurement
•
Mercury Porosimetry for pore size distribution
•
Mercury Porosimetry for volume (%) porosity measurement
•
Micropore analysis for pore volume, area and size measurement
•
Bulk (envelope) density measurement
•
Analysis of powder blends
•
Analysis of solid dosage forms
At MCA Services we offer a complete suite of techniques for the
characterisation of sample porosity with the experience and expertise to
actively assist with the interpretation of results and their relation to
development and performance criteria. Our ongoing in-house research
continues to highlight the significance of and application of these analytical
techniques to the pharmaceutical sector.


01763262333
LATEST RESEARCH NEWS:
New research paper by MCA Services
BET surface area measurement of
commercial magnesium stearate by krypton
adsorption in preference to nitrogen
adsorption. International Journal of
Pharmaceutics. Volume 568, 10 September
2019.